ozone chemistry
- Standard State: gas
- Boling temperature: -111.9 °C
- Melting temperature: -195.5 °C
- Specific Gravity: 2.144
- Formula: O3
- Colour: Pale Blue
- Occurs: naturally in troposphere
- Formed: From nitrogen oxides and organic oxides emitted as a product of combustion engines or from passing an electric current through air.
The name ozone comes from the Greek ozein, for “to smell”. It is an allotropic form of oxygen having three atoms in each molecule, formula O3.
It is, in standard state, a pale blue, highly poisonous gas with a strong odour. Liquid ozone is a deep blue and strongly magnetic.
Ozone is formed when an electric spark is passed through oxygen, and causes a detectable odour near electrical machinery. The commercial method of preparation consists of passing cold, dry oxygen through a silent electrical discharge.
Ozone is much more active chemically than ordinary oxygen and is a better oxidising agent. It is used in purifying water, sterilising air, and bleaching certain foods.
Ozone formed in the atmosphere is from nitrogen oxides and organic gases emitted by automobiles and industrial sources, however, is a health hazard, and it may cause serious crop damage in some areas.
The structure of ozone has 3 oxygen atoms, but steric hindrance prevents it from forming a triangular structure, with each O atom forming the expected 2 bonds. Instead each Oxygen forms only 1 bond, with the remaining negative charge being spread throughout the molecule.
Ozone Reactions
Ozone reactions mostly take place in the atmosphere, as the unstable molecule reacts in sunlight. These are described in Low Level Ozone and High Level Ozone. There are a group of compounds called Ozonides, which are formed during the reaction of ozone with alkali metal hydroxides, formally containing the O3– ion. An ozonide is also the unstable compound formed by the addition of ozone to the C=C bond in alkenes. Ozone is also a very strong oxidising agent. This is shown below:
2Fe2++2H++ O3  2Fe3++H2O+O2
2Fe3++H2O+O2
Ozonolysis is a reaction of alkenes with ozone. Ozone can be produced in the lab using a machine called an ozonator. This ozone is passed through a solution of the alkene, first producing an ozonide. This ozonide is subsequently reduced, giving the result of cleavage around the double bond, with an oxygen attached to each carbon from the double bond. The reaction is shown below:
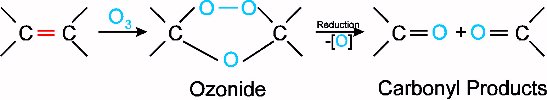
It becomes apparent that as well as being important in environmental chemistry, ozone also plays a role in organic chemistry

