acid rain
Essay on Acid Rain: Definition, Causes, Adverse Effects and Control!
The term ‘Acid Rain’ was first used by Robert A. Smith in 1872 from his studies of air in Manchester, England. The widespread occurrence of acid rain was recognized only in 1980. Acid rain is a rain or any other form of precipitation that is unusually acidic, i.e., elevated levels of hydrogen ions (low pH).
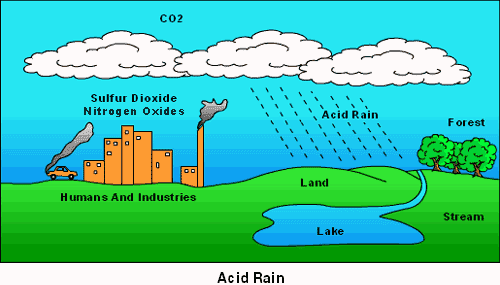
What we call acid rain is the oxides of sulphur and nitrogen originating from industrial operations and fossil fuel combustion, the major sources of acid forming gases combine with the water in the air. Acid forming gases are oxidized over several days by which time they travel several thousand kilometres.
In the atmosphere these gases are ultimately converted into sulphuric and nitric acids. This acidic mixture then falls as rain, sleet, mist or snow or as solid flakes. Hydrogen chloride emission forms hydrochloric acid. These acids cause acidic rain.

Rain water is turned acidic when its pH falls below 5.6. In fact clean or natural rain water has a pH of 5.6 at 20°C because of formation of carbonic acid due to dissolution of CO2 in water. Parts of India such as North East, Coastal regions of Kerela, Orissa, Bihar, and West Bengal have reported decline in fertility of soil due to reduced pH of soil (increased in acidity). Acid rain is the phenomenon of wet and dry acidic deposition.
Wet deposition:
Wet deposition of acids occurs when any form of precipitation (rain, snow, and so on) removes acids from the atmosphere and delivers it to the Earth’s surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosols are both of importance for wet deposition.
Dry deposition:
Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition. This occurs when particles and gases stick to the ground, plants or other surfaces.
During the last few decades acid rain occurred within the downwind of areas of major industrial areas in Europe and America. Emissions of sulphur dioxide (SO2) into sulphate or NO2 into nitrate particles and by combining with water vapour into mild sulphuric or nitric acids and return to earth as dew, drizzle, fog, sleet, snow, and rain is the mechanism of acid rain. Acid rain is a rain or any other form of precipitation that is unusually toxic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals, and infrastructure.
Acid rain is caused by emissions of sulphur dioxide and nitrogen acids, which react with the water, smell molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulphur dioxide into the atmosphere with positive results.
Nitrogen oxides can also be produced naturally by lightning strikes and sulphur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.
“Acid u” is a popular term referring to the deposition of wet (rain, snow, sleet, fog, cloud water, and dew) and dry (acidifying particles and gases) acidic components. Distilled water, once carbon dioxide is removed, has a neutral pH of 7. Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are alkaline.
“Clean” or unpolluted rain has an acidic pH, but usually no lower than 5.7, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid. However, unpolluted rain can also contain other chemicals which affect its pH. A common example is nitric acid produced by electric discharge in the atmosphere such as lightning. Carbonic acid is formed by the reaction.
H2O (1) + CO2 (g) → H2CO3 (aq)
Carbonic acid then can ionize in water forming low concentrations of hydronium and carbonate ions:
H2O (1) + H2CO3 (aq) <==> HCO–3 <==> (aq) + H3O+ (aq)
Acid deposition as an environmental issue would include additional acids to H2CO3.
Causes of Acid Rain:
Acid deposition can occur via natural sources like volcanoes but it is mainly caused by the release of sulphur dioxide and nitrogen oxide during fossil fuel combustion. When these gases are discharged into the atmosphere they react with the water, oxygen, and other gases already present there to form sulphuric acid, ammonium nitrate, and nitric acid. These acids then disperse over large areas because of wind patterns and fall back to the ground as acid rain or other forms of precipitation.
The gases responsible for acid deposition are normally a by-product of electric power generation and the burning of coal. As such, it began entering the atmosphere in large amounts during the Industrial Revolution and was first discovered by a Scottish chemist, Robert Angus Smith, in 1852.
In that year, he discovered the relationship between acid rain and atmospheric pollution in Manchester, England. Although it was discovered in the 1800s, acid deposition did not gain significant public attention until the 1960s and the term acid rain was coined in 1972.
Adverse Effects:
Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms as well as causing damage to buildings and having impacts on human health.
1. Surface waters and aquatic animals:
Both the lower pH and higher aluminium concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pH lower than 5 most fish eggs will not hatch and lower pHs can kill adult fish.
As lakes and rivers become more acidic biodiversity is reduced. Acid rain has eliminated insect life and some fish species, including the brook trout in some lakes, streams, and creeks in geographically sensitive areas, such as the Adirondack Mountains of the United States.
However, the extent to which acid rain contributes directly or indirectly via runoff from the catchments to lake and river acidity (i.e., depending on characteristics of the surrounding watershed) is variable.
The United States Environmental Protection Agency’s (EPA) website states: “Of the lakes and streams surveyed, acid rain caused acidity in 75 percent of the acidic lakes and about 50 percent of the acidic streams”.
As the lake becomes more acidic the fish find it more difficult to reproduce successfully. It is not only the acid in the water that kills them, but also poisonous minerals like aluminium that are washed out of the surrounding ground into the water. The birds that eat the fish also begin to suffer as the harmful minerals build up inside.
2. Soils:
Soil biology and chemistry can be seriously damaged by acid rain. Some microbes are unable to tolerate changes to low pH and are killed. The enzymes of these microbes are denatured (changed in shape so they no longer function) by the acid. The hydronium ions of acid rain also mobilize toxins such as aluminium, and leach away essential nutrients and minerals such as magnesium.
2 H+ (aq) + Mg2+ (clay) <==> 2H+ (clay) + Mg2+ (aq)
Soil chemistry can be dramatically changed when base cations, such as calcium and magnesium, are leached by acid rain thereby affecting sensitive species, such as sugar maple (Acer saccharum).
Acid rain can damage soil by destroying many vital substances and washing away the nutrients. Soils naturally contain small amounts of poisonous minerals such as mercury and aluminium.
Normally these minerals do not cause serious problems, but when acid rain falls on the ground and the acidity of the soil increases, chemical reactions occur allowing the poisonous minerals to be taken up by the plant roots. The trees and plants are then damaged and any animals eating them will absorb the poisons, which will stay in their bodies.
3. Forests and other vegetation:
The acid takes important minerals away from the leaves and the soil. Without these minerals, trees and plants cannot grow properly. Damaged trees lose their leaves, have stunted growth and damaged bark. This makes it easier for fungi and insects to attack the tree, and as a result the tree may die.
Acid rain not only damages soil but can also affect the trees directly. Pollutants can block or damage the little pores on the leaves through which the plant takes in the air it needs to survive. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.
Other plants can also be damaged by acid rain, but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death.
4. Human health effects:
Acid rain does not directly affect human health. The acid in the rainwater is too dilute to have direct adverse effects. However, the particulates responsible for acid rain (sulphur dioxide and nitrogen oxides) do have an adverse effect. Increased amounts of fine particulate matter in the air do contribute to heart and lung problems including asthma and bronchitis.
5. Other adverse effects on Monuments:
Acid rain can also damage buildings and historic monuments and statues, especially those made of rocks, such as limestone and marble that contain large amounts of calcium carbonate.
When sulphur pollutants fall on to buildings made from limestone and sandstone they react with minerals in the stone to form a powdery substance that can be washed away by rain. Acids in the rain react with the calcium compounds in the stones to create gypsum, which then flakes off.
CaCO3 (s) + H2SO4 (aq) <==> CaSO4 (aq) + CO2 (g) + H2O (1)
The effects of this are commonly seen on old gravestones, where acid rain can cause the inscriptions to become completely illegible. Acid rain also increases the corrosion rate of metals, in particular iron, steel, copper and bronze. Famous buildings like the Statue of Liberty in New York, the Taj Mahal in India and St. Paul’s Cathedral in London have all been damaged by this sort of air pollution.
Acid rain can also damage stained glass windows in churches, railway lines and steel bridges. The acid rain slowly eats away them all. Building materials crumble away, metals are corroded.
Control of Acid Rain:
1. Emission of SO2 and NO2 from industries and power plants should be reduced by using pollution control equipment’s such as scrubbers in the smokestacks of factories. These spray a mixture of water and limestone into the polluting gases, recapturing the sulphur.
2. Liming of lakes and soils should be done to correct the adverse effects of acid rain.
3. A coating of protective layer of inert polymer should be given in the interior of water pipes for drinking water.
4. In catalytic converters, the gases are passed over metal coated beads that convert harmful chemicals into less harmful ones.

